
On May 14th, Amiens University Medical Center published a study paper named “Dynamic profile for the detection of anti-SARS-CoV-2 antibodies using four immunochromatographic assays” on the authoritative journal Journal of Infection. The study adopted 4 immunochromatographic assays including Biotime product to conduct dynamic antibody detection research on confirmed patients with COVID-19 infection. According to the study report, we can draw below conclusions:
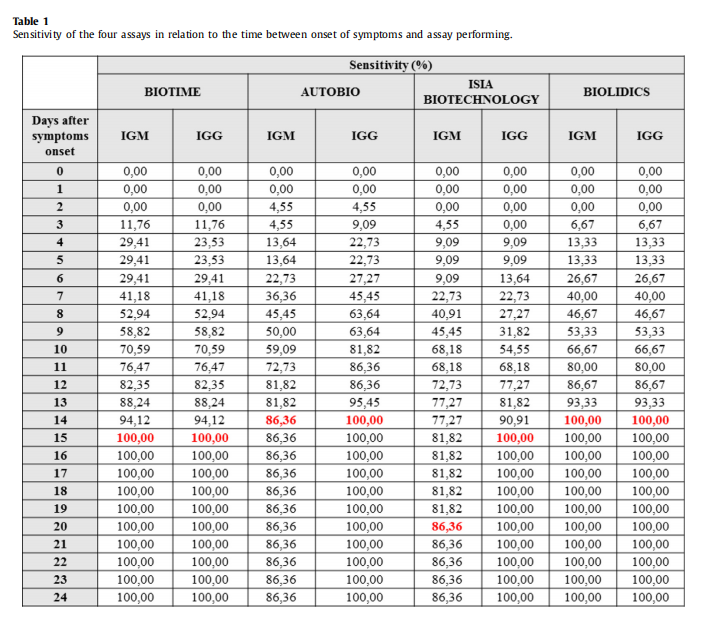
Conclusion 1:The data shows that the sensitivity of our product is great among 4 reagents.

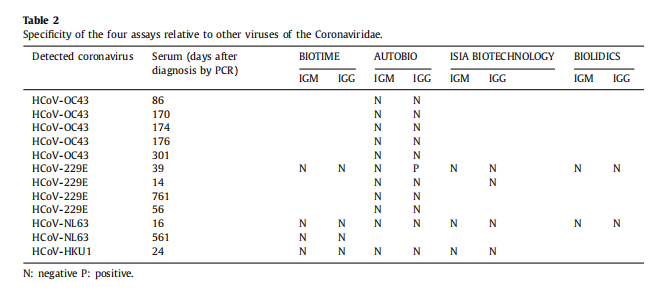
Conclusion 2: The specificity of our product is strong and it got good results in cross-reactivity study.
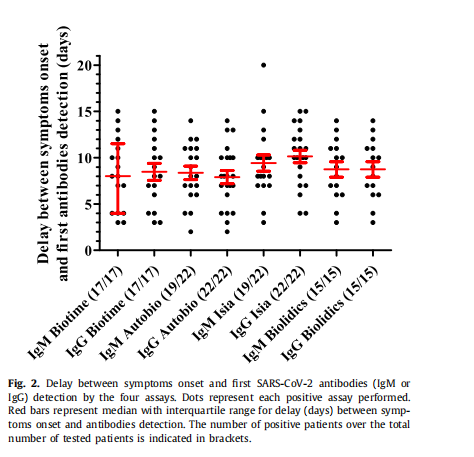
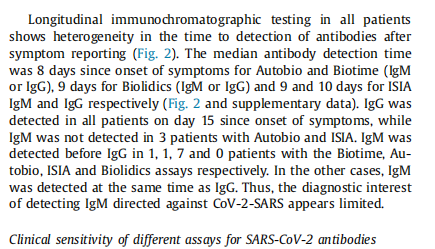
Conclusion 3: The median antibody detection time of Biotime product after appearing symptom is the eighth day, which is better than comparative products in the ninth day.
Biotime SARS-CoV-2 IgG/IgM Rapid Qualitative Test has been done lots of verification experiments in domestic and foreign clinical and research institutions. Besides, it has got many registration certificates including CE, MHRA, Russia, Ukraine, Brazil, Philippines, Ecuador, Chile and Honduras, etc, and it has been sold into different countries such as Australia, Germany, France, Philippines, New Zealand, and UK, etc.
Reference: http://www.elsevier.com/locate/jinf
Dynamic profifile for the detection of anti-SARS-CoV-2 antibodies using four immuno- chromatographic assays
© 2016 Xiamen Biotime Biotechnology Co., Ltd. all rights reserved